Why The Absorbance Increase And Decrease As The Concentration Increase
There will therefore be more active sites available to catalyse the substrate so there will be a higher frequency of successful collisions in a higher concentration. In the previous post on free radical substitution reactions we talked about why heat or light is required in free-radical reactions.

Beer Lambert Law An Overview Sciencedirect Topics
Beer S Law Theoretical Principles

Absorbance Measurements Bmg Labtech
IR absorbance spectroscopy obeys the BeerLambert law whereby band absorbance is linearly related to concentration of the molecule in solution.

Why the absorbance increase and decrease as the concentration increase. Accordingly the Beer-Lambert law which states a linear relation of absorbance and the concentration of an absorbing molecule is theoretically not applicable for OD 600 measurements. In this post were going to go through the mechanism of a free-radical substitution reaction which has three key types of steps. Fortunately absorption and scattering of radiation by the flame are corrected by analyzing a blank.
We quantified forestation and deforestation as an increase or decrease in forest cover from 2000 to 2010 respectively at each 5 x 5 km cell across the globe. Of a bacterial culture is thus primarily not an absorbance. Synthetic substrates that release a fluorescent dye in an enzyme-catalyzed reaction are also available such as 4-methylumbelliferyl-β-D-galactoside for assaying β-galactosidase or 4-methylumbelliferyl-butyrate for assaying Candida rugosa lipase.
Perform a Q-test on the class data and then calculate the average percent Al by weight in alum the standard deviation of the data and. Concentration effects the absorbance very similarly to path length. Divide the moles of solute by the volume of the solution in liters.
How does activators affect enzyme activity. Higher enzyme concentration extract yielded higher absorbance increases indicating higher rate. UV -Visible spectroscopy Absorption of light in the UVVisible part of the spectrum 210 900 nm.
Concentrationwe will call this a Beers law plotis a straight line with a y-intercept of zero and a slope of ab or varepsilon b. A valid. One system checks the decrease in absorbance at 340 mm when the ammonia reacts with alpha-ketoglutaric acid.
If the concentration of solution is increased then there are more molecules for the light to hit when it passes through. However if the resting Ca 2 concentration inside the muscle cells becomes too large the muscles will contract without the internal nerve signal to trigger an increase in the concentration. From the equation for the best-fit line for the absorbance at 525 nm as a function of Al 3 determine the percent aluminum by weight in alum and share your results with the class.
Enzyme activators are chemical compounds that increase a velocity of enzymatic reaction. Normally this increase in Ca 2 concentration is triggered by a nerve impulse. In practice however it has been shown that despite of the absence of absorbance the Beer-Lambert can be used for low density cultures.
1 Graph of absorbance vs time with varying enzyme concentration. Of pigment in solution will be greater so the solution appears darker 3 so we use same volume of water so that results from different temp are comparable as they should be the only variable to affect the results not water. For instance muscle contraction is initiated by a sudden increase in calcium concentration in the muscle cells.
As the concentration increases there are more molecules in the solution and more light is blocked. The 5 x 5 km cells represented the local scale of analysis both for forest cover and for the climatic variables LST ET and albedo. Herein how do you find the concentration of an unknown solution.
Students should use values from their own graphs. Take samples periodically to monitor both the decrease in the starch concentration and the increase in the reducing sugars until most of the starch is hydrolyzed. Spectral interferences also occur when components of the samples matrix other than the analyte react to form molecular species such as oxides and hydroxides.
In summary for a UV TiO 2 process an increase in the solar concentration ratio of 1 to 2 suns gave rise to an increase 13 in the degradation of the pollutant levels from 41 to 54 while for a H 2 O 2 UV TiO 2 process an increase in the solar concentration ratio of 1 to 2 suns led to an increase 13 in the degradation of the pollutant levels of 79 to 92 respectively. This allows the estimation of the association constant and thereby ΔG by measuring the decrease in the intensity of the free AH band on formation of an H-bond along with knowledge of the total amounts of each substance present. When the concentration of the sample is too low to be measured using a standard 10mm optical path length cell and concentration is difficult in cases where the sample vaporizes or undergoes a chemical or molecular structural change during the concentration process then a cell with a longer optical path length is used to enable the optimum absorption sensitivity of absorption measurement.
This is why on the 14th day of the fermentation process the content of alcohol decreased and there was an increase in acidity as well as the production of organic acids including acetic acid. K m k on B then A surfaceA bulk and the binding rate can be expressed as 111 The surface concentration of the free binding site B is the difference between the concentration of the complex at saturation AB max and the. The transitions that result in the absorption of.
Fundamental chemical and instrumental. 1ml extract had the highest R2 value of 09792. In this example M 045 mol04 L 1125 M.
The starch concentration is measured with the same steps outlined above and the sugar concentration with the dinitrosalicylic colorimetric method used in the previous experiment. 1 if too much water the concentration of pigment in solution will be lower so the solution will appear lighter. If the mass transport rate is much larger than the association rate constant or if the surface concentration of the immobilized species is low ie.
Set up your equation so the molarity M molV where mol is the number of moles of the solute and V is the volume of the solvent. The Astra system measures the rate of increase in conductivity of the solution in which urea is hydrolyzed. In some cases a Beers law plot deviates from this ideal behavior see Figure 1029 and such deviations from linearity are divided into three categories.
Solution of unknown concentration is 0219. Another name people tend to use for a logarithmic relationship factor of. Determine the molarity of the solution.
Acetic acid which is the dominating acid present in a fermented solution contributes to the decrease in pH from 5 to as low as 3 10 28. Initiation Propagation and Termination In Free Radical Reactions. Beers law suggests that a plot of absorbance vs.
An increase of 1 unit on the absorbance scale translates into a factor of 10 decrease on the T scale. Increase but at around 4060 C the percentage transmission decreases sharply. Even though the test is now performed mostly.
2 if too little water the conct. The result is an apparent increase in the samples absorbance. Initiation propagation and termination.
Solve the equation and label the answer M. Oxidation reactions can therefore be followed by a decrease in fluorescence and reduction reactions by an increase. When there is a higher concentration enzyme there is more of an enzyme packed into an area.
Rest of the in-depth answer is here. At lower temperatures the tonoplast and plasmalemma are intact and betalain molecules are. At higher temperatures the rate of decrease usually levels out.
Drops as wave length increase. After the reaction has proceeded for several minutes does the amount of catalyst increase decrease or remain the same. Nominal volumes of the volumetric glassware used in this exercise.
Thats why we prefer to determine concentration of DNA at 600nm using spectrophotometer. Absorbance molar absorptivity.
Solved Beer S Law States That As The Concentration Of A Chegg Com

The Decrease In Absorbance Of Alr With Time A The Increase In The Download Scientific Diagram
Spectrophotometry Cellbiologyolm

Two Selective Spectrophotometry Methods For The Determination Of Thioridazine Hydrochloride In Tablets And In Biological Fluids
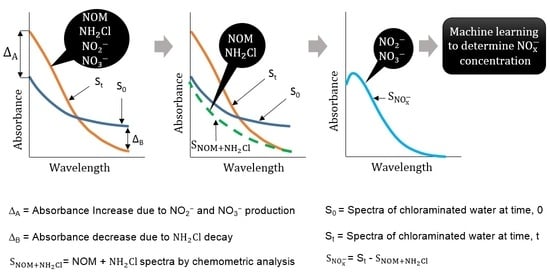
Sensors Free Full Text Development Of An Optical Method To Monitor Nitrification In Drinking Water Html

Standard Curve Showing The Decrease In Absorbance Of A Tb Solution At Download Scientific Diagram
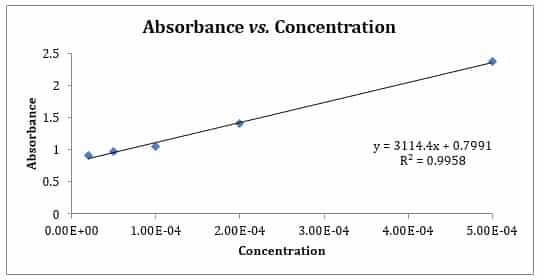
Beer S Law Lab Explained Absorbance Vs Concentration Schoolworkhelper

Substrate Concentration An Overview Sciencedirect Topics


Comments
Post a Comment